New Salmonella and Listeria Outbreaks Added to FDA Watchlist

The U.S. Food and Drug Administration (FDA) has announced updates to several ongoing foodborne illness investigations, with two new outbreaks added to the national watchlist. Officials stress that while not every recall or alert results in confirmed illnesses, the agency is actively monitoring emerging threats that affect consumers across the country, including here in Florida.
According to the FDA’s Coordinated Outbreak Response and Evaluation (CORE) Network, a new outbreak of Salmonella Lomalinda (reference #1339) and a new outbreak of Listeria monocytogenes (reference #1334) have been added to the federal outbreak investigation table. Additionally, case counts are climbing in several ongoing outbreaks:
- Salmonella Enteritidis (ref #1329) rose from 39 to 40 cases.
- Cyclospora cayetanensis (ref #1325) jumped from 51 to 67 cases.
- Cyclospora cayetanensis (ref #1313) increased from 41 to 47 cases.
The FDA explains that outbreak investigations can be at different stages. Some, like the newly reported outbreaks, still lack enough information to pinpoint a specific food source, while others further down the list are nearing completion. If investigators do identify a product linked to illnesses, the agency will issue a public health advisory with clear guidance for consumers.
This is the information currently available through the FDA on the active investigations:
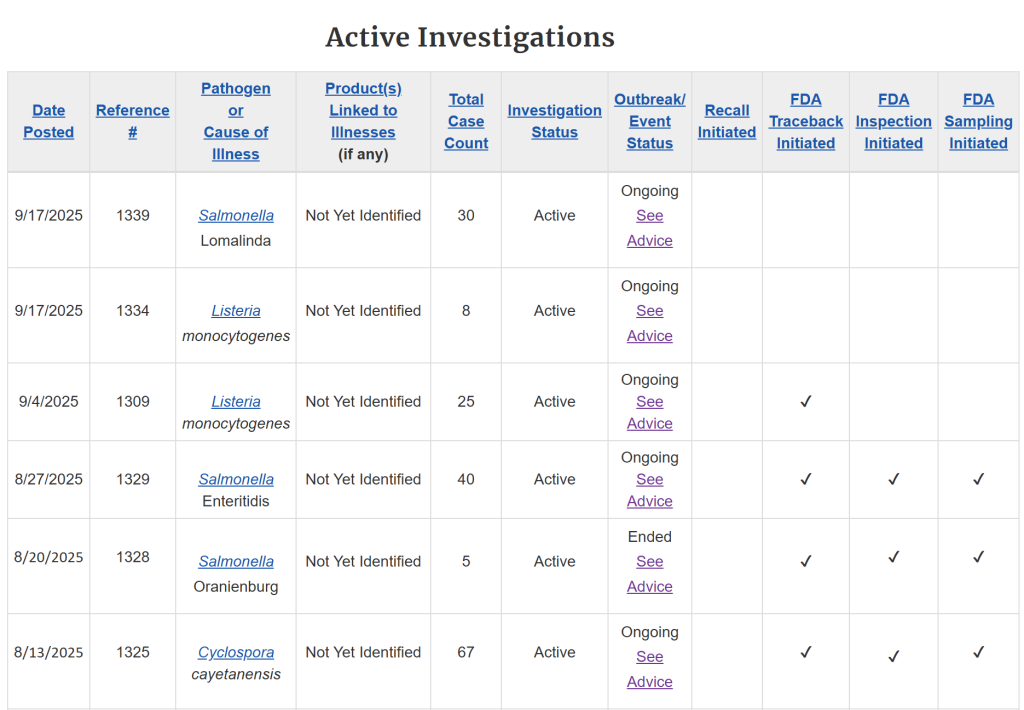
While some investigations may not lead to concrete findings, the FDA commits to releasing summaries if contributing factors or causes are identified that can help prevent future outbreaks.
Food safety reminders for consumers and businesses during outbreaks:
- Stay up to date with the FDA’s recall and safety alert notices.
- Practice safe food handling, especially with fresh produce, meats, and ready-to-eat products.
- Retailers and shippers are encouraged to follow the FDA’s food safety resources during active investigations.
For the most current details on active outbreak investigations, visit the FDA’s CORE outbreak investigation table.






